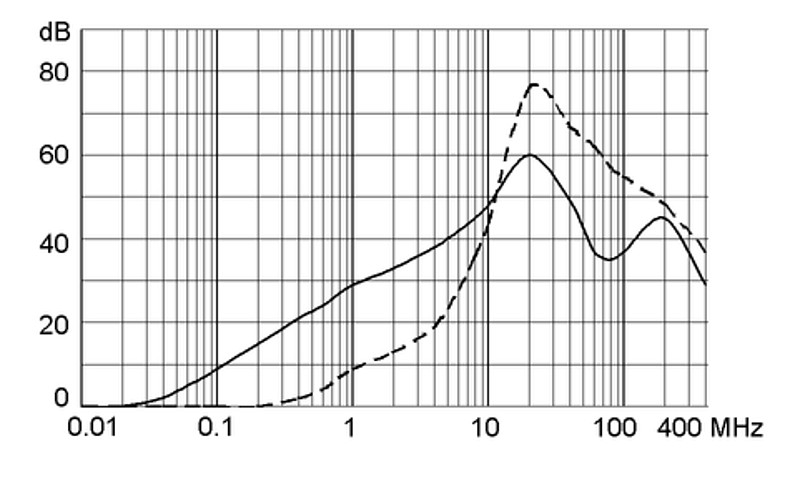
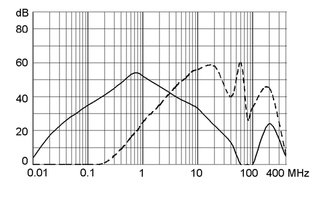
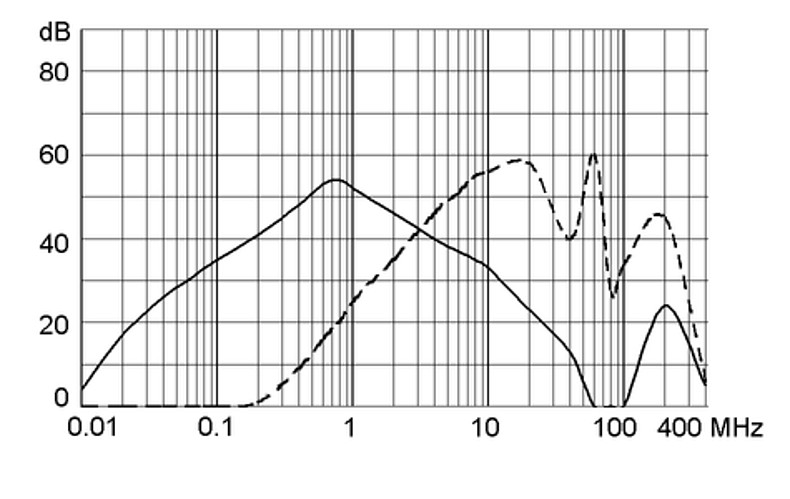
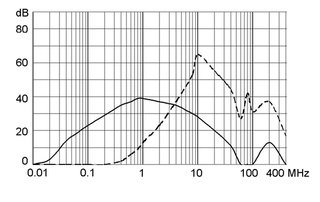
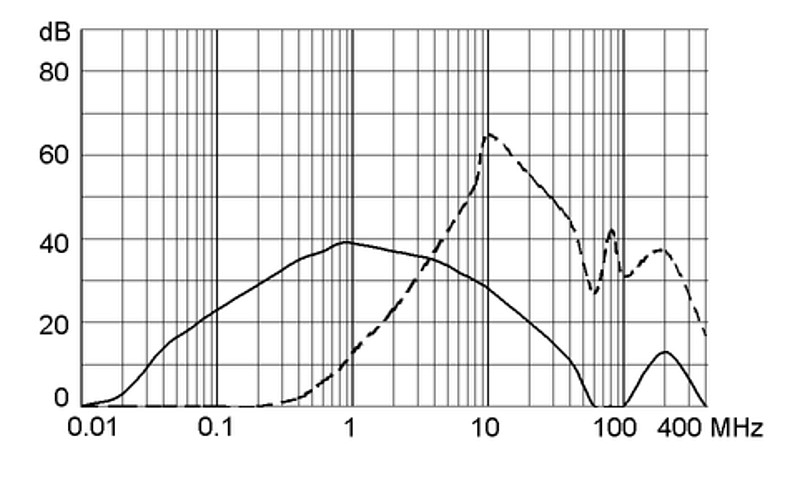
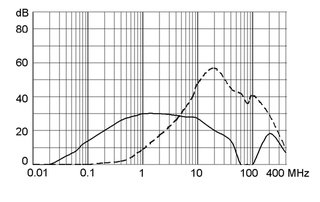
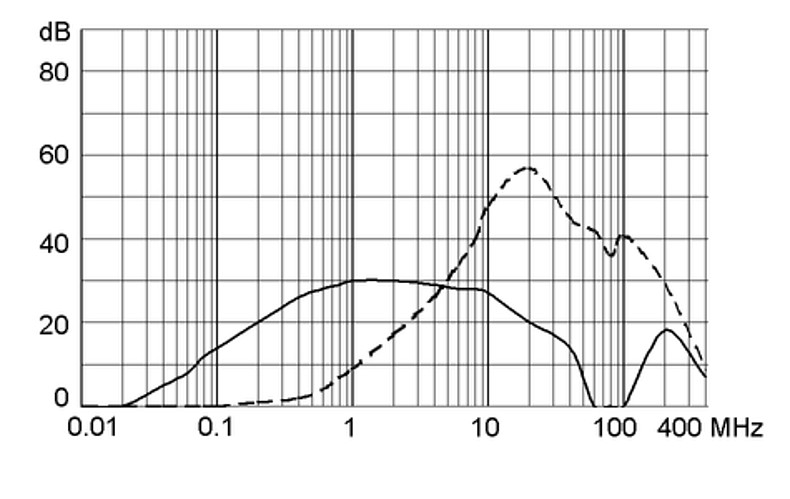
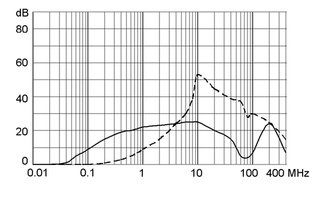
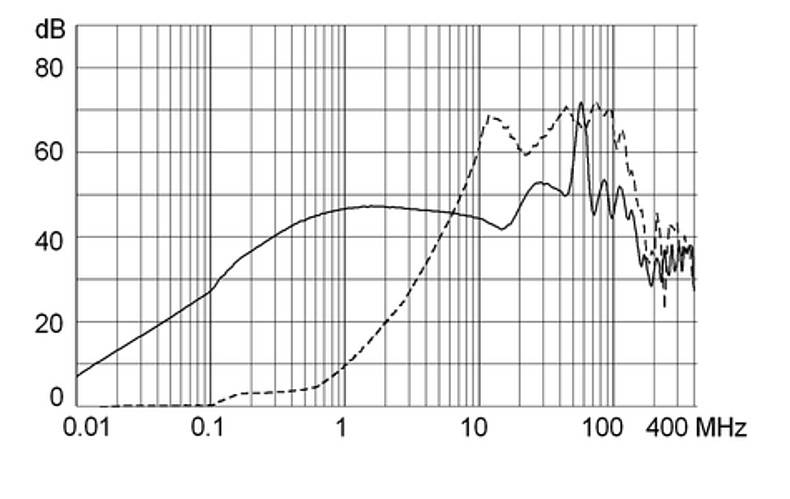
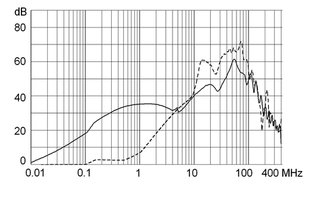
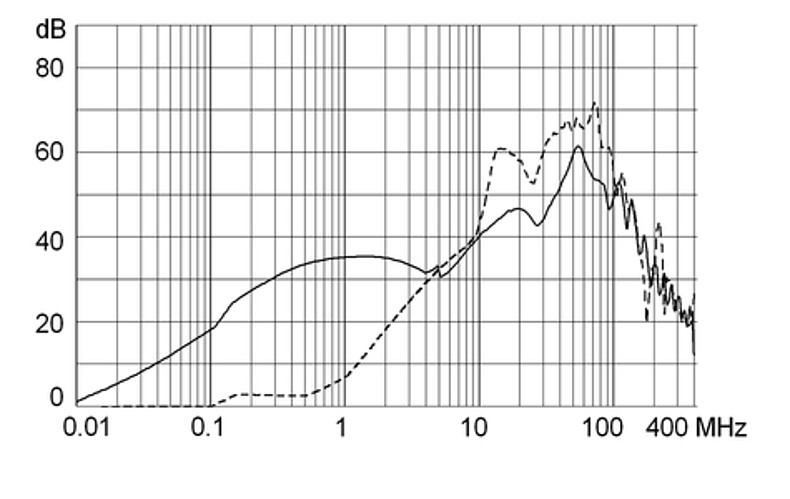
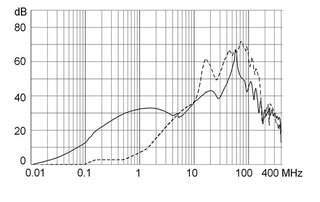
Ratings IEC
The electrical characteristics describe the approved operating values for the state of rated current and rated voltage considering the mentioned conditions.
General information on electrical characteristics colors of fuses
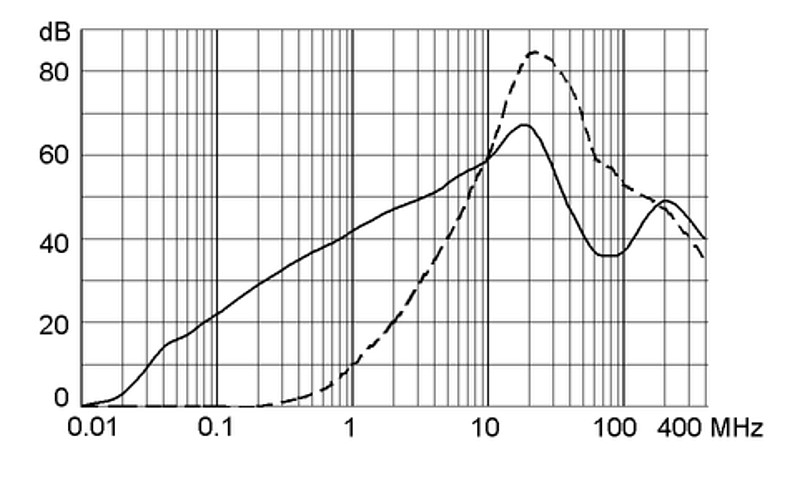
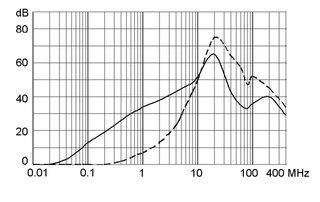
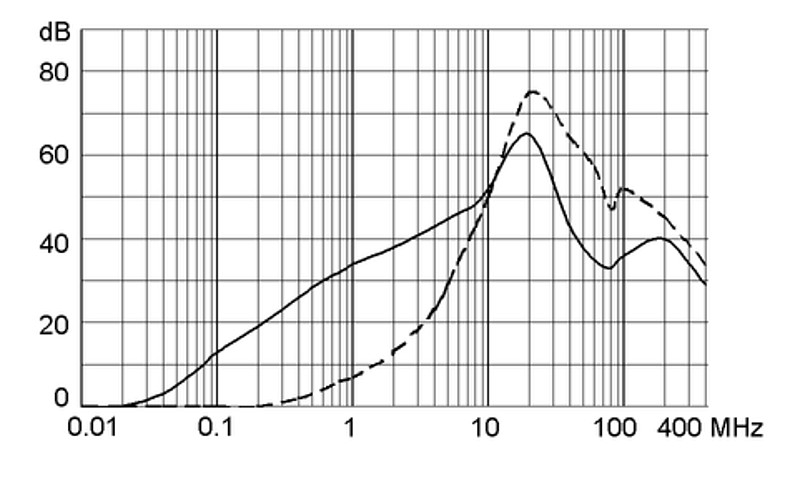
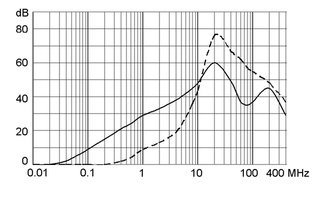
Ratings IEC, UL/CSA General information on the rated current and the rated voltage at EMC products
Rated Voltage
Rated current The values are different depending on the approval body and therefore are referenced in the corresponding admission cards for the respective product families. Details about the approvals and ratings are listed in the data sheets.
Search approval information for different families
Approvals


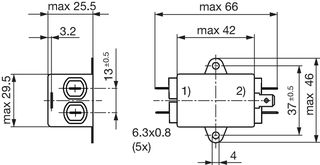
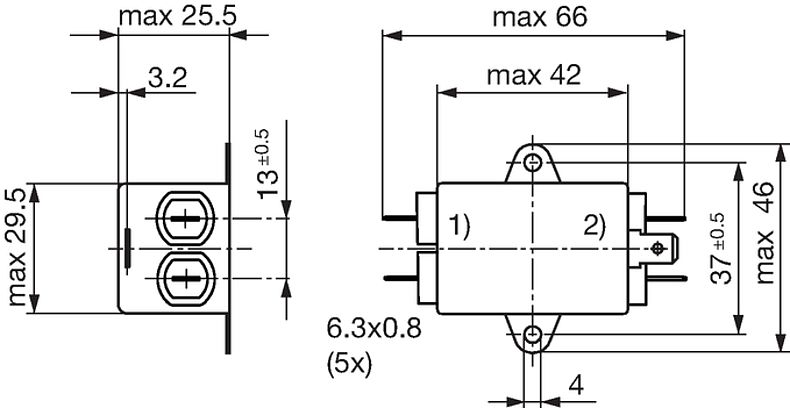
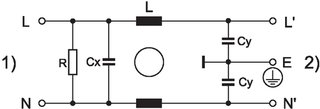
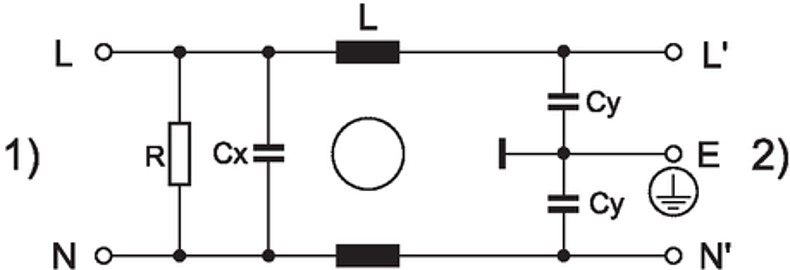
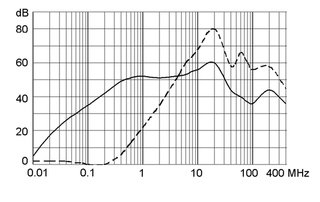
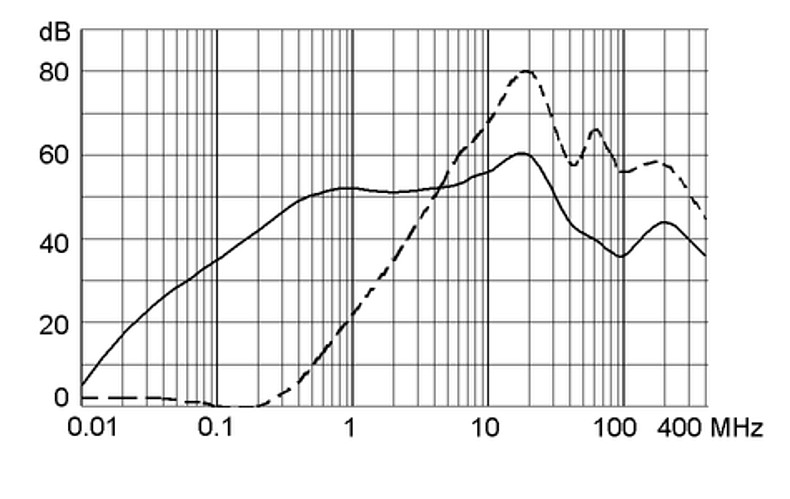
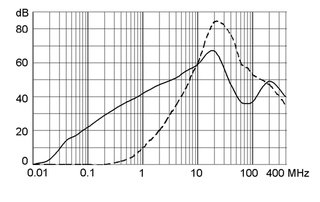
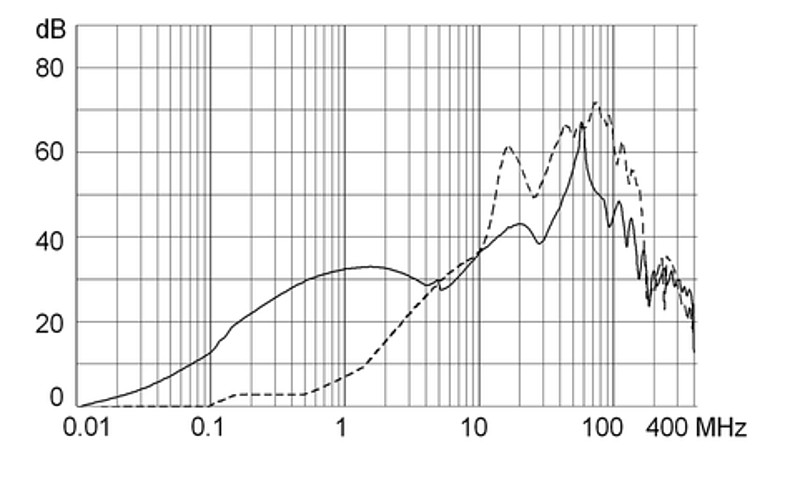
 Technical Data
Technical Data